Abstract
Introduction: Hemophagocytic lymphohistiocytosis (HLH) is a frequently fatal severe inflammatory syndrome. HLH evaluations are conducted by multiple inpatient services due to the protean manifestations of the disease in adults. We observed difficulty identifying HLH precipitating conditions, hemorrhage, and erroneous testing as impediments to optimal care. We embarked on a HLH quality improvement project to examine strategies of precipitant identification, quantify hemorrhagic complications, and decrease erroneous test ordering.
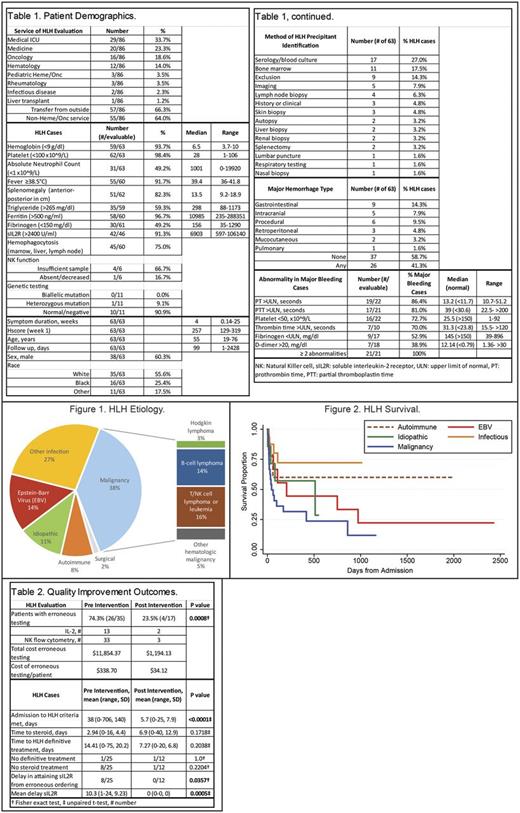
Methods: The Johns Hopkins Institutional Review Board approved this single-institution retrospective review. Patients were identified from billing and laboratory databases at The Johns Hopkins Hospital. Patients were included if they were evaluated for HLH during an inpatient hospitalization by their treating clinicians between January 1, 2009 and July 30, 2016. Patients <18 years of age at the time of evaluation were excluded. Clinical, laboratory, and imaging findings were obtained by chart review (SAM). HLH was defined as patients meeting at least 5 of 8 HLH-2004 criteria (Henter et al 2007); Hscore during the first inpatient week was also computed (Fardet et al 2014).
Interventions occurred in 2015 and consisted of: 1) a review of HLH diagnostic testing, 2) presentation of findings to hematology staff, 3) development of an evidence-based HLH consult template, and 4) consultation on suspected HLH cases. Ordering interleukin-2 levels (IL2) or quantitative natural killer cell (NK) flow cytometry, instead of soluble interleukin-2 receptor (sIL2R) or NK functional assays were defined as erroneous testing in HLH evaluations. Test costs were obtained from Quest Diagnostics. Test ordering between 2014-2016 were compared before and after the interventions.
Practices identifying the HLH precipitant were determined by chart review. Major hemorrhage was defined as bleeding 1) into a critical site, 2) requiring modification of blood product support, 3) requiring procedural intervention, or 4) leading to death. Coagulation parameters were examined in the 24hrs before hemorrhage was clinically evident. Overall survival was defined as date of hospital admission until death. Descriptive statistics were computed with percentages and frequencies. Survival analysis was performed with STATA14. Quality metrics were evaluated with either Fisher's exact test or unpaired t-test; statistical analysis used two-tailed significance testing and P<0.05.
Results: During the study period, 86 patients were evaluated for HLH, and 63 fulfilled HLH-2004 diagnostic criteria (Table 1). Most patients (64%) were initially admitted to a non-hematology/oncology service. Serologic testing and bone marrow biopsy were most likely to identify the HLH-precipitating illness in 27% and 17.5% of cases, respectively. Major hemorrhage occurred in 41.3% of patients and occurred at the time of at least two coagulation abnormalities. HLH median survival ranged from 45 days with malignancy to not reached with autoimmune diseases and non-EBV infections (Figures 1 & 2).
The interventions significantly decreased the occurrence of erroneous testing from 74.3% to 23.5% of patients (P=0.0008), and decreased costs of erroneous testing from $338 to $34 per patient during the evaluation period (Table 2). The interventions also significantly reduced the time to fulfillment of HLH criteria (38 vs 5.7 days, P<.0001), and reduced the delay in obtaining sIL2R testing after initial erroneous ordering (10.3 vs 0 days, P=0.0005). There were no significant differences in time to steroid administration, time to definitive HLH treatment, or in patient number without treatment.
Conclusions: Adults with HLH initially present to non-hematology/oncology inpatient services. Diagnostic uncertainty, hemorrhage, and erroneous testing impair patient care. We observed that serological testing and bone marrow biopsy were most likely to identify the HLH precipitant. Major hemorrhage was common and occurred when multiple coagulation abnormalities were evident. Our quality improvement project significantly decreased erroneous testing and improved time to diagnosis. With these data, we are developing an internal HLH decision support tool to facilitate identification of HLH precipitant illness, curtail erroneous testing, and minimize morbidity from major hemorrhage.
Merrill: True North Therapeutics: Consultancy. Streiff: Roche: Research Funding; Portola: Research Funding; Janssen Scientific Affairs, LLC: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding. Lanzkron: HRSA: Research Funding; Bayer: Research Funding; Global Blood Therapeutics: Research Funding; Pfizer: Research Funding; PCORI: Research Funding; Prolong: Research Funding. Brodsky: Alexion Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Grant Funding.
Author notes
Asterisk with author names denotes non-ASH members.